
BACKGROUND
In paroxysmal nocturnal hemoglobinuria (PNH), intravascular hemolysis (IVH) is mediated by the membrane attack complex, while extravascular hemolysis (EVH) is facilitated by C3 opsonization. Although eculizumab (ECU), a C5 inhibitor, inhibits IVH, ~70% of patients remain anemic and 36% require ≥1 transfusion per year due to C3-mediated EVH. Pegcetacoplan (APL-2), a C3 inhibitor, has the potential to control both IVH and EVH in PNH.
AIMS
PEGASUS, a phase 3, randomized, open-label, controlled trial (NCT03500549), assessed the efficacy and safety of pegcetacoplan compared to ECU in patients with PNH.
METHODS
Eighty patients aged ≥18 years with a confirmed diagnosis of PNH, hemoglobin levels <10.5 g/dL (despite stable ECU for ≥3 months), reticulocytes >1.0 × ULN, platelets >50 × 109/L and neutrophils >0.5 × 109/L were included. All patients provided written informed consent and completed a run-in period of 4 weeks with pegcetacoplan plus ECU before 1:1 randomization to monotherapy with pegcetacoplan (41 patients, 1080 mg subcutaneously twice a week) or ECU (39 patients, continuing current dosing regimen). The primary endpoint was change in hemoglobin level from baseline (start of run-in period) to week 16. Key secondary and secondary endpoints were hemoglobin normalization (defined as hemoglobin level greater than or equal to lower limit of normal range) in the absence of transfusions, transfusion avoidance, absolute reticulocyte counts, lactate dehydrogenase (LDH), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score, and adverse events (AEs). Hierarchical significance testing for secondary efficacy endpoints was gated on the success of the primary efficacy endpoint. Post hoc analyses included hemoglobin stabilization (defined as avoidance of a >1 g/dL decrease from baseline) in the absence of transfusions.
RESULTS
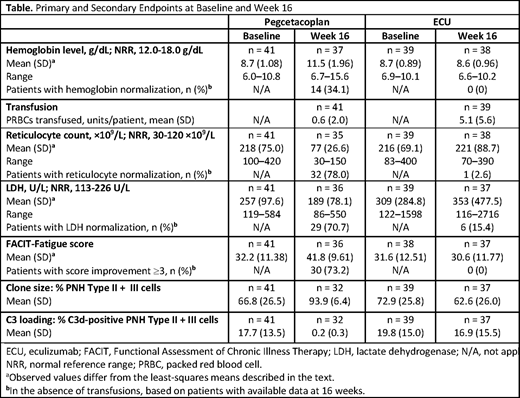
Pegcetacoplan demonstrated superiority to ECU in change in hemoglobin level at week 16, with an adjusted treatment difference of 3.84 g/dL (p<0.0001). The least-squares (LS) mean (SE) changes were 2.37 (0.36) g/dL with pegcetacoplan and −1.47 (0.67) g/dL with ECU, both changed from baseline of 8.7 g/dL (Table). At week 16, a greater proportion of patients receiving pegcetacoplan achieved ≥2 g/dL improvement in hemoglobin (61% vs 0%), hemoglobin normalization (34% vs 0%), and hemoglobin stabilization (85% vs 15%) in the absence of transfusion as compared with ECU. Transfusion avoidance was achieved in 35 (85.4%) patients with pegcetacoplan vs 6 (15.4%) patients with ECU, an adjusted risk difference of 62.5% (95% CI, 48.3-76.8), demonstrating non-inferiority. Non-inferiority was also shown for absolute reticulocyte counts, which decreased with pegcetacoplan and slightly increased with ECU (LS mean [SE] changes of −136 [6.5] and 28 [11.9] 109/L, respectively). LS mean (SE) changes in LDH were −15 (42.7) and −10 (71.0) U/L, respectively. LS mean (SE) FACIT-Fatigue score increased with pegcetacoplan (9.2 [1.61]) and decreased with ECU (−2.7 [2.82]). As the change in LDH did not meet statistical non-inferiority, changes in FACIT-Fatigue score were not tested for non-inferiority due to prespecified hierarchical testing. Thirty-six of 41 (87.8%) patients with pegcetacoplan and 34/39 (87.2%) with ECU reported an AE; 7/41 (17.1%) and 6/39 (15.4%), respectively, had serious AEs. Most AEs were mild. AEs included injection site reactions (pegcetacoplan, 15/41 [36.6%]; ECU, 1/39 [2.6%] patients) and diarrhea (pegcetacoplan, 9/41 [22.0%]; ECU, 1/39 [2.6%]); infections were reported in 12/41 (29.3%) and 10/39 (25.6%) patients, respectively. By week 16, breakthrough hemolysis was reported in 4 (9.8%) patients with pegcetacoplan and 9 (23.1%) with ECU, leading to discontinuation in 3 patients on pegcetacoplan.
CONCLUSIONS
In this phase 3 trial in patients with PNH, pegcetacoplan demonstrated superiority to ECU in hemoglobin level, and improved clinical outcomes at week 16 with transfusion avoidance in most patients. The safety profile of pegcetacoplan was comparable to that of ECU. The efficacy of pegcetacoplan validates the prevention of extravascular as well as intravascular hemolysis in PNH, leading to a potential new therapeutic option.
Hillmen:Acerta: Other: Financial or material support; AbbVie: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Roche: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Gilead: Other: Financial or material support, Research Funding; Alexion: Consultancy, Research Funding, Speakers Bureau; Apellis: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Pharmacyclics: Other: Financial or material support, Research Funding; Janssen: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau. Szer:Prevail Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy; Takeda: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Weitz:Apellis: Consultancy, Honoraria; Alexion: Consultancy, Honoraria, Speakers Bureau. Röth:Biocryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Research Funding. Hoechsmann:Alexion: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Panse:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Grunenthal: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chugai: Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees. Usuki:Apellis: Research Funding; Chugai: Research Funding; Novartis: Research Funding, Speakers Bureau; Alexion: Research Funding, Speakers Bureau. Griffin:Biocryst: Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Honoraria, Other: Conference Support. Kiladjian:Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. de Castro:Biocryst: Honoraria, Other: Data monitoring committee; Novartis: Honoraria, Other: Steering committee; Alexion: Honoraria, Research Funding; Apellis: Consultancy, Honoraria, Research Funding. Tan:Apellis: Consultancy, Patents & Royalties. Hamdani:Apellis: Current Employment, Current equity holder in publicly-traded company. Deschatelets:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Francois:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Grossi:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Risitano:Jazz: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Samsung: Membership on an entity's Board of Directors or advisory committees; Amyndas: Consultancy; RA pharma: Research Funding; Biocryst: Membership on an entity's Board of Directors or advisory committees; Apellis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Achillion: Membership on an entity's Board of Directors or advisory committees; Pfizer: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alnylam: Research Funding; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Peffault De Latour:Apellis: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Pegcetacoplan is an investigational drug for the treatment of paroxysmal nocturnal hemoglobinuria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal